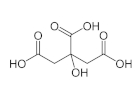
Citric acid is also known as lemon salt among the public; It is a weak, organic carboxylic acid. It is a hygroscopic chemical and can be found both in pure form and as hydrates. It is also found naturally in many animals and plants. Lemons can contain up to 8% citric acid. It is a widely used acid and is used as a pH regulator, sweetener and binder for binding ions or molecules to metal surfaces. Citric acid, which is a monohydrate, can be converted to anhydrous at 78 ° C.
Areas of use:
Since citric acid is both easy to use and relatively strong, it is used very often, especially as a food additive. Today, more than half of the citric acid produced is consumed for this purpose. In addition, it is a very good sequestering agent for metals. It is used to prevent the formation of lime in boilers and pipes. At the same time, when applied to metals, it ensures that cleaners foam better and work better. In this way, there is no need for a softening process for water. 6% solutions give very good results in removing hard water marks, especially on surfaces such as glass. It can also be used as a rust remover in steel production and to passivate stainless steels.
In addition, citric acid is also used as an affordable pH regulator in the cosmetics sector, paints, photography and many other areas.
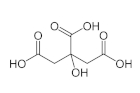
Citric acid is also known as lemon salt among the public; It is a weak, organic carboxylic acid. It is a hygroscopic chemical and can be found both in pure form and as hydrates. It is also found naturally in many animals and plants. Lemons can contain up to 8% citric acid. It is a widely used acid and is used as a pH regulator, sweetener and binder for binding ions or molecules to metal surfaces. Citric acid, which is a monohydrate, can be converted to anhydrous at 78 ° C.
Areas of use:
Since citric acid is both easy to use and relatively strong, it is used very often, especially as a food additive. More than half of the citric acid produced today is consumed for this purpose. In addition, it is a very good sequestering agent for metals. It is used to prevent the formation of lime in boilers and pipes. At the same time, when applied to metals, it ensures that cleaners foam better and work better. In this way, there is no need for a softening process for water. 6% solutions give very good results in removing hard water marks, especially on surfaces such as glass. It can also be used as a rust remover in steel production and to passivate stainless steels.
In addition, citric acid is also used as an affordable pH regulator in the cosmetics sector, paints, photography and many other areas.
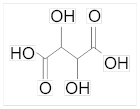
Tartaric acid is found in many fruits such as grapes, tamarind, bananas and citrus fruits. Tartaric acid is found in many sour vegetables such as tomatoes. Its IUPAC name is 2,3-dihydroxybutanedioic acid. It is found in nature as acidic. In addition to being found in nature in free form, the most common ones are seen as Calcium Tartrate, Potassium Tartrate and Sodium Tartrate salts. It has a low toxicity level compared to other acids such as citric acid or acetic acid. It is insoluble in water; however, it is well soluble in alcohols, ethers and glycols at room temperature.
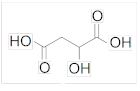
It is a chemical naturally found in all fruits. It is formed during fruit metabolism. Malic acid is an organic dicarboxylic acid that acts as a tart flavoring in many sour foods or tarts. It has been found to increase exercise tolerance in people with fibromyalgia and chronic fatigue. It supports energy production and helps the reactions in the body to proceed. It is a chemical that provides cells with energy and carbon skeletons for the formation of amino acids. It is the source of excessive sourness. In sour desserts, if we want to obtain a low level of sour taste, it is used together with citric acid or instead of citric acid and malic acid. It has an important role in the metabolism of carbohydrates, that is, in the energy production mechanisms for cells. Esters and salts of Malic Acid are called malates. The most commonly used type is Magnesium Malate, which is a magnesium salt. Malic acid formula: C4H6O5 E Code: E 296







